
100 points if you help quickly
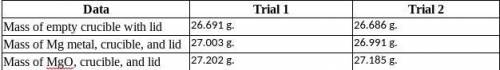
Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for each trial.
Trial 1:
Trial 2:
Determine the percent yield of MgO for your experiment for each trial.
Trial 1:
Trial 2:
Determine the average percent yield of MgO for the two trials.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
You know the right answer?
100 points if you help quickly
Magnesium is the limiting reactant in this experiment. Calculate the...
Questions


History, 09.03.2021 18:40

History, 09.03.2021 18:40

Mathematics, 09.03.2021 18:40

Mathematics, 09.03.2021 18:40



Physics, 09.03.2021 18:40


Mathematics, 09.03.2021 18:40







Mathematics, 09.03.2021 18:40



Mathematics, 09.03.2021 18:40



