

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1
You know the right answer?
If 45.00 g of precipitate is formed from the reaction of 0.100 mol/L
HCL(aq) and 0.124 mol/L AgNO3(...
Questions

Biology, 15.04.2021 21:50

Mathematics, 15.04.2021 21:50


Arts, 15.04.2021 21:50


English, 15.04.2021 21:50


Health, 15.04.2021 21:50

Mathematics, 15.04.2021 21:50

Mathematics, 15.04.2021 21:50



English, 15.04.2021 21:50

Mathematics, 15.04.2021 21:50




Biology, 15.04.2021 21:50


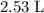 (rounded to three significant figures) assuming that
(rounded to three significant figures) assuming that 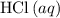 is in excess.
is in excess. 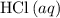 and
and 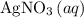 precipitate,
precipitate, 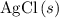 (the said precipitate) and
(the said precipitate) and 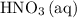 are produced:
are produced: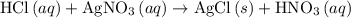 (verify that this equation is indeed balanced.)
(verify that this equation is indeed balanced.)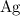 and
and 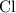 on a modern periodic table:
on a modern periodic table: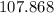 .
.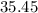 .
.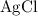 :
: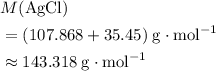 .
. of this compound:
of this compound: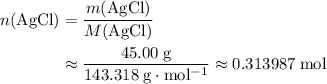 .
. and
and 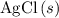 are both one.
are both one. 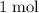 of
of 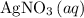 formula units to produce
formula units to produce 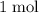 of
of 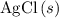 formula units.
formula units. 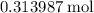 of
of 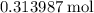 of
of 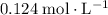 :
: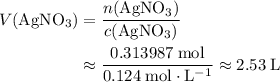 .
.


