
Chemistry, 20.01.2021 22:50 amandajbrewerdavis
14.A 4.25 gram sample of an unknown gas is found to occupy a volume of 1.70 L at a pressure of 883 mm Hg and a temperature of 58 °C. The molar mass of the unknown gas is g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1


Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1
You know the right answer?
14.A 4.25 gram sample of an unknown gas is found to occupy a volume of 1.70 L at a pressure of 883 m...
Questions

Arts, 23.11.2020 01:00


Social Studies, 23.11.2020 01:00

Law, 23.11.2020 01:00



Social Studies, 23.11.2020 01:00


Computers and Technology, 23.11.2020 01:00

Law, 23.11.2020 01:00

Mathematics, 23.11.2020 01:00

Chemistry, 23.11.2020 01:00




Chemistry, 23.11.2020 01:00

Social Studies, 23.11.2020 01:00

Medicine, 23.11.2020 01:00

Chemistry, 23.11.2020 01:00

Mathematics, 23.11.2020 01:00

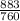 = 1.16atm
= 1.16atm 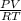 =
= 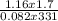 = 0.07mole
= 0.07mole 

