

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
13.How many grams of phosphorus (P4) are needed to completely consume 79.2 L of chlorine gas accordi...
Questions

Business, 20.11.2020 19:30

World Languages, 20.11.2020 19:30


Mathematics, 20.11.2020 19:30

English, 20.11.2020 19:30



Mathematics, 20.11.2020 19:30







Mathematics, 20.11.2020 19:30





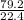 = 3.54mole
= 3.54mole 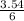 = 0.59mole of P₄
= 0.59mole of P₄ 

