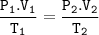Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
Suppose that 25,0 mL of a gas at 725 mmHg and 298K is converted to
273'K and 760 mmHg atm. What wou...
Questions

Biology, 27.06.2019 17:30






Chemistry, 27.06.2019 17:30

Mathematics, 27.06.2019 17:30

English, 27.06.2019 17:30

English, 27.06.2019 17:30








Mathematics, 27.06.2019 17:30

Computers and Technology, 27.06.2019 17:30

History, 27.06.2019 17:30