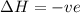Chemistry, 20.01.2021 23:50 kefernanda40
A student made a sketch of a potential energy diagram to represent a reaction with a −ΔH value. A curve line graph is shown. The y axis of the graph has the title Potential Energy and kJ written in parenthesis. The x axis of the graph has the title Reaction Pathway. The curve begins at a higher level and ends at a slightly lower level. A broken horizontal line is shown from a point labelled X on the y axis to the point where the curve begins. Another broken horizontal line is shown from a point labeled Y on the y axis to the point where the curve ends. Explain, using complete sentences, why the diagram made by the student is correct or incorrect. Be sure to also explain what the values of X and Y represent.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1


Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
A student made a sketch of a potential energy diagram to represent a reaction with a −ΔH value. A cu...
Questions




Biology, 19.03.2021 23:00

Mathematics, 19.03.2021 23:00

Health, 19.03.2021 23:00

Mathematics, 19.03.2021 23:00

History, 19.03.2021 23:00

Geography, 19.03.2021 23:00




Mathematics, 19.03.2021 23:10

Mathematics, 19.03.2021 23:10


Mathematics, 19.03.2021 23:10



Mathematics, 19.03.2021 23:10

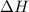 for the reaction comes out to be positive.
for the reaction comes out to be positive.