
--What do you have to do to the coefficients of equation I below to get to equation II?
i) 2 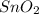 + 4
+ 4 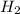 --> 2 Sn + 4
--> 2 Sn + 4 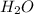
ii) 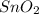 + 2
+ 2 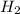 --> Sn + 2
--> Sn + 2 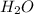
--Can you divide equation II by another factor and still have it be correct? Why or why not?
--In a complete sentence, write down a method you could use to determine if an equation is written in the correct way.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

You know the right answer?
--What do you have to do to the coefficients of equation I below to get to equation II?
i) 2 + 4...
Questions


Mathematics, 13.07.2021 17:10

Mathematics, 13.07.2021 17:10


Mathematics, 13.07.2021 17:10

Mathematics, 13.07.2021 17:10



Mathematics, 13.07.2021 17:10




Computers and Technology, 13.07.2021 17:10


History, 13.07.2021 17:10

History, 13.07.2021 17:10

Mathematics, 13.07.2021 17:10


Business, 13.07.2021 17:10

English, 13.07.2021 17:10



