
Chemistry, 21.01.2021 08:50 raymond5799
8. For each of the following pairs of atoms, highlight the atom that will attract shared electrons more
strongly.
a. Carbon or
Chlorine
b. Rubidium or Bromine
C. lodine or Indium
d. Silver
Sulfur
e. Arsenic
or
Sodium
f. Hydrogen or Selenium
or
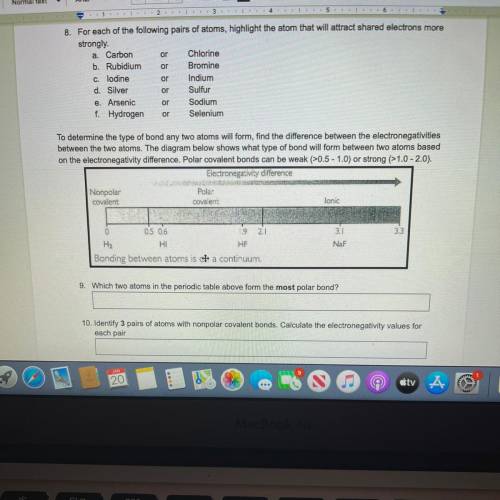
To determine the type of bond any two atoms will form, find the difference between the electronegativities
between the two atoms. The diagram below shows what type of bond will form between two atoms based
on the electronegativity difference. Polar covalent bonds can be weak (>0.5 - 1.0) or strong (>1.0-2.0).
Electronegativity difference
Nonpolar
Polas
covalent
covalent
lonic
0
2
3.3
0.5 0.6
9
H2
HI
HF
Bonding between atoms is a continuum.
3.1
NaF
9. Which two atoms in the periodic table above form the most polar bond?
10. Identify 3 pairs of atoms with nonpolar covalent bonds. Calculate the electronegativity values
each pair

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
8. For each of the following pairs of atoms, highlight the atom that will attract shared electrons m...
Questions













English, 11.11.2020 17:30


Geography, 11.11.2020 17:30



Mathematics, 11.11.2020 17:30


Social Studies, 11.11.2020 17:30



