
Chemistry, 21.01.2021 15:40 cxttiemsp021
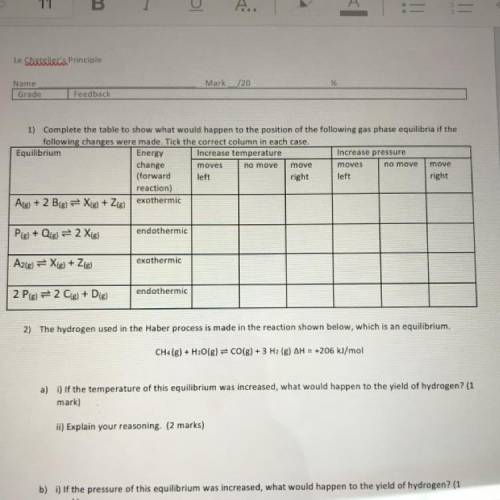
1) Complete the table to show what would happen to the position of the following gas phase equilibria if the
following changes were made. Tick the correct column in each case.
Equilibrium
Energy Increase temperature
Increase pressure
change
moves
moves
move
(forward left
right left
right
reaction)
Ale) + 2 B(g) = X(g) + Zig) exothermic
endothermic
Ple) + Qig) = 2 Xig)
exothermic
A2(g) = X(g) + 2(g)
endothermic
2 Pig) = 2 C(s) + D(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1

Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3

Chemistry, 23.06.2019 05:30
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1
You know the right answer?
1) Complete the table to show what would happen to the position of the following gas phase equilibri...
Questions

Mathematics, 10.10.2019 18:00



Mathematics, 10.10.2019 18:00

Biology, 10.10.2019 18:00

Mathematics, 10.10.2019 18:00


Chemistry, 10.10.2019 18:00







English, 10.10.2019 18:00

History, 10.10.2019 18:00

Chemistry, 10.10.2019 18:00


English, 10.10.2019 18:00



