

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
A student titrates 0.2087 g of an unknown diprotic acid with 0.1224 M sodium hydroxide. It takes 32....
Questions





Social Studies, 18.03.2021 02:20



Mathematics, 18.03.2021 02:20


Mathematics, 18.03.2021 02:20


Biology, 18.03.2021 02:20

Mathematics, 18.03.2021 02:20

Mathematics, 18.03.2021 02:20



Mathematics, 18.03.2021 02:20


Mathematics, 18.03.2021 02:20

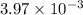 moles.
moles. 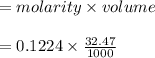
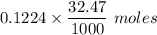
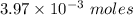 .
.

