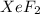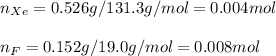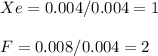Chemistry, 21.01.2021 22:00 shongmadi77
A compound containing xenon and fluorine was prepared by shining sunlight on a mixture of Xe (0.526 g) and excess F2 gas. If you isolate 0.678 g of the new compound, what is its empirical formula

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

You know the right answer?
A compound containing xenon and fluorine was prepared by shining sunlight on a mixture of Xe (0.526...
Questions

Mathematics, 12.02.2021 20:30

Mathematics, 12.02.2021 20:30

Mathematics, 12.02.2021 20:30




Mathematics, 12.02.2021 20:30

Social Studies, 12.02.2021 20:30


English, 12.02.2021 20:30


Mathematics, 12.02.2021 20:30

Mathematics, 12.02.2021 20:30


Mathematics, 12.02.2021 20:30

Mathematics, 12.02.2021 20:30




Mathematics, 12.02.2021 20:30