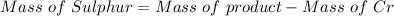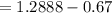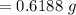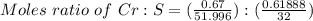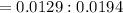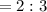Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

You know the right answer?
A 0.67 gram sample of chromium is reacted with sulfur. The resulting chromium sulfide has a mass of...
Questions








Physics, 27.06.2019 22:00

Mathematics, 27.06.2019 22:00



Biology, 27.06.2019 22:00


Biology, 27.06.2019 22:00



History, 27.06.2019 22:00

Social Studies, 27.06.2019 22:00

Mathematics, 27.06.2019 22:00

Mathematics, 27.06.2019 22:00

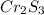 ".
".