
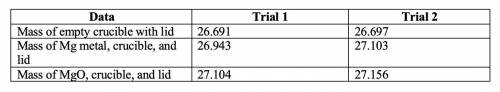
1. Write the balanced chemical equation for the reaction you are performing.
2. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of Mg, crucible, and lid (row 2 in the chart) to find the mass of magnesium for each trial.
• Trial 1: 0.252
• Trial 2: 0.406
3. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of MgO, crucible, and lid (row 3 in the chart) to find the mass of magnesium oxide for each trial. This is the actual yield of magnesium oxide for each trial.
• Trial 1: 0.413
• Trial 2: 0.459
4. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for each trial.
• Trial 1:
• Trial 2:
5. Determine the percent yield of MgO for your experiment for each trial.
• Trial 1:
• Trial 2:
6. Determine the average percent yield of MgO for the two trials.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1
You know the right answer?
1. Write the balanced chemical equation for the reaction you are performing.
2. Subtract the mass o...
Questions

Mathematics, 29.08.2020 21:01


Mathematics, 29.08.2020 21:01


Mathematics, 29.08.2020 21:01

Social Studies, 29.08.2020 21:01


Mathematics, 29.08.2020 21:01




Computers and Technology, 29.08.2020 21:01










