
Chemistry, 22.01.2021 06:00 willoughbysierra
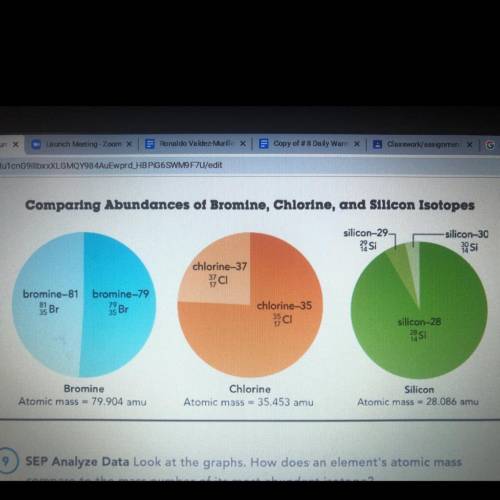
Look at the graphs. How does an element's atomic mass compare to the mass number of its most abundant isotope?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1
You know the right answer?
Look at the graphs. How does an element's atomic mass compare to the mass number of its most abundan...
Questions

Computers and Technology, 17.12.2019 01:31





Biology, 17.12.2019 01:31


Computers and Technology, 17.12.2019 01:31

Physics, 17.12.2019 01:31

Computers and Technology, 17.12.2019 01:31



Computers and Technology, 17.12.2019 01:31









