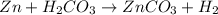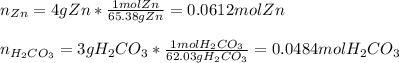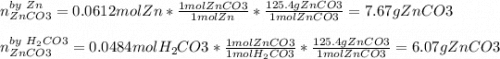Chemistry, 22.01.2021 08:30 davidtemple
1. Calculate the mass of the product, if you started with 4 g of Zn and 3 g of H2CO3.
Zn + H2CO3
ZnCO2 + H2 * I’ll cash app for the right answer plus explanation”


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2


Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1

Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
You know the right answer?
1. Calculate the mass of the product, if you started with 4 g of Zn and 3 g of H2CO3.
Zn + H2CO3
Questions


Mathematics, 15.06.2021 06:00




Mathematics, 15.06.2021 06:00

Mathematics, 15.06.2021 06:00

Mathematics, 15.06.2021 06:00


Mathematics, 15.06.2021 06:00

Mathematics, 15.06.2021 06:00


English, 15.06.2021 06:00

Mathematics, 15.06.2021 06:00


Mathematics, 15.06.2021 06:00


Biology, 15.06.2021 06:00

Mathematics, 15.06.2021 06:00