
Chemistry, 22.01.2021 16:00 robbiegfarmer
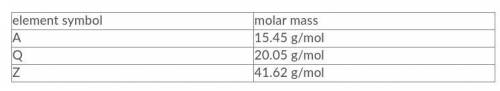
Three elements, A, Q, and Z, have the molar masses indicated in the table below. Given a compound with a composition of 42.9% A, 18.6% Q and 38.5% Z by mass, what is the empirical formula of this compound?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3


Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1

Chemistry, 23.06.2019 14:00
How are casts formed by decaying organisms? organisms turn into rock over time. organisms leave carbon residue on a rock. organisms leave impressions in sediment that hardens into rock. impressions left by organisms are filled in with sediment that hardens into rock.
Answers: 2
You know the right answer?
Three elements, A, Q, and Z, have the molar masses indicated in the table below. Given a compound wi...
Questions


Business, 02.07.2019 05:30



Mathematics, 02.07.2019 05:40

Mathematics, 02.07.2019 05:40

Spanish, 02.07.2019 05:40


English, 02.07.2019 05:40

English, 02.07.2019 05:40

English, 02.07.2019 05:40

Mathematics, 02.07.2019 05:40

Mathematics, 02.07.2019 05:40

Geography, 02.07.2019 05:40

Geography, 02.07.2019 05:40

Mathematics, 02.07.2019 05:40

Chemistry, 02.07.2019 05:40

History, 02.07.2019 05:40

History, 02.07.2019 05:40

Mathematics, 02.07.2019 05:40



