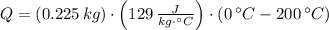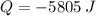Chemistry, 22.01.2021 18:30 jarednash015
What is the total amount of heat needed to change 2250 g of silver at 200.0°C to 0.0°C? The specific heat of silver is 0.129 J/g∙°C

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is lincoln's purpose in writing this speech? question 1 options: to stress the difficulties of war to honor those who died in the war to call for an end to the war to call the country to join a new war
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

You know the right answer?
What is the total amount of heat needed to change 2250 g of silver at 200.0°C to 0.0°C? The specific...
Questions

Mathematics, 23.12.2021 15:10

Mathematics, 23.12.2021 15:10

Physics, 23.12.2021 15:10


Business, 23.12.2021 15:10



Physics, 23.12.2021 15:20

Social Studies, 23.12.2021 15:20



Mathematics, 23.12.2021 15:20

SAT, 23.12.2021 15:20



Computers and Technology, 23.12.2021 15:30



Computers and Technology, 23.12.2021 15:30

English, 23.12.2021 15:30

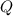 ), measured in joules, is determined by the following formula:
), measured in joules, is determined by the following formula: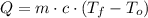 (1)
(1)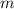 - Mass, measured in kilograms.
- Mass, measured in kilograms. - Specific heat of silver, measured in joules per grams-degrees Celsius.
- Specific heat of silver, measured in joules per grams-degrees Celsius.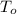 ,
, 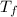 - Initial and final temperatures, measured in degrees Celsius.
- Initial and final temperatures, measured in degrees Celsius. 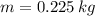 ,
, 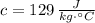 ,
, 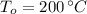 and
and 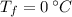 , then the heat received by the sample of silver is:
, then the heat received by the sample of silver is: