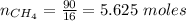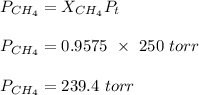Chemistry, 22.01.2021 20:00 ashleybashaam6821
A mixture of 90.0 grams of CH4 and 10.0 grams of argon has a pressure of 250 torr under conditions of constant temperature and volume. The partial pressure of CH4 in tore is?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1

Chemistry, 23.06.2019 15:00
What is the mass in grams of 0.94 moles of sodium bicarbonate, nahco3?
Answers: 1

Chemistry, 23.06.2019 21:30
How does changing the surface area increase the reaction rate of a chemical change
Answers: 1
You know the right answer?
A mixture of 90.0 grams of CH4 and 10.0 grams of argon has a pressure of 250 torr under conditions o...
Questions

Chemistry, 17.07.2019 17:30


Arts, 17.07.2019 17:30

Arts, 17.07.2019 17:30



Arts, 17.07.2019 17:30

Spanish, 17.07.2019 17:30

Computers and Technology, 17.07.2019 17:30

Health, 17.07.2019 17:30

Computers and Technology, 17.07.2019 17:30








Computers and Technology, 17.07.2019 17:30