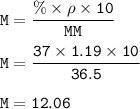Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1
You know the right answer?
A bottle of a commercial reagent-grade hydrochloric acid, HCI, has the following
information on its...
Questions


Mathematics, 05.03.2021 18:50



History, 05.03.2021 18:50


Law, 05.03.2021 18:50

Mathematics, 05.03.2021 18:50



Mathematics, 05.03.2021 18:50

Mathematics, 05.03.2021 18:50



Spanish, 05.03.2021 18:50


Mathematics, 05.03.2021 18:50


Mathematics, 05.03.2021 18:50

Mathematics, 05.03.2021 18:50