
Calculate the ph for each of the following cases in the titration of 50.0 ml of 0.210 m hclo(aq) with 0.210 m koh(aq).
(a) before addition of any koh
(b) after addition of 25.0 ml of koh
(c) after addition of 35.0 ml of koh
(d) after addition of 50.0 ml of koh
(e) after addition of 60.0 ml of koh

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1


Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
Calculate the ph for each of the following cases in the titration of 50.0 ml of 0.210 m hclo(aq) wit...
Questions

Biology, 22.06.2020 15:57

Biology, 22.06.2020 15:57


Biology, 22.06.2020 15:57

Mathematics, 22.06.2020 15:57

Mathematics, 22.06.2020 15:57



Physics, 22.06.2020 15:57


English, 22.06.2020 15:57

English, 22.06.2020 15:57

Chemistry, 22.06.2020 15:57

Mathematics, 22.06.2020 15:57

Mathematics, 22.06.2020 15:57





English, 22.06.2020 15:57

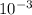
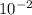
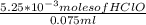 = 0.070M
= 0.070M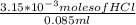 = 0.037M
= 0.037M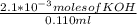 = 0.020M
= 0.020M

