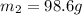Chemistry, 04.02.2020 11:54 tamikeen2243
To a sample of water at 23.4oc in a constant pressure calorimeter of negligible heat capacity is added a 12.1 g piece of aluminium whose temperature is 81.7oc. if the final temperature of water is 24.9 oc, calculate the mass of the water in the calorimeter. ans: 98.6g
-i know that the specific heat of aluminum is 0.900 j/g ‡ ác
- _t al is 24.9ác _ 81.7ác = _56.8ác
- _twater and _tcalorimeter are both 24.9ác _ 23.4ác = 1.5ác.
-the specific heat of water is 4.184 j/g ‡ ác.
but i tried using m=q/s_t. i'm really stuck, can anyone me?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1


Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
To a sample of water at 23.4oc in a constant pressure calorimeter of negligible heat capacity is add...
Questions


Biology, 29.01.2020 21:52

Mathematics, 29.01.2020 21:52


Physics, 29.01.2020 21:52


Biology, 29.01.2020 21:52


Mathematics, 29.01.2020 21:52

Mathematics, 29.01.2020 21:52

Mathematics, 29.01.2020 21:52

English, 29.01.2020 21:52

Arts, 29.01.2020 21:52

History, 29.01.2020 21:52



History, 29.01.2020 21:52



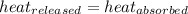
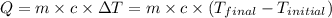
![-m_1\times c_1\times (T_{final}-T_1)=[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0500/2981/27fc4.png) .................(1)
.................(1)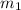 = mass of aluminium = 12.1 g
= mass of aluminium = 12.1 g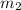 = mass of water = ?
= mass of water = ?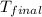 = final temperature =
= final temperature = 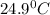
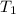 = temperature of aluminium =
= temperature of aluminium = 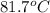
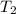 = temperature of water =
= temperature of water = 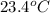
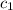 = specific heat of aluminium =
= specific heat of aluminium = 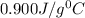
 = specific heat of water=
= specific heat of water= 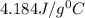
![-12.1\times 0.900\times (24.9-81.7)=-[m_2\times 4.184\times (24.9-23.4)]](/tpl/images/0500/2981/9ba9b.png)