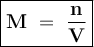A chemist prepares a solution of potassium iodide (KI) by measuring out 88. umol of potassium iodide into a 200. mL volumetric flask and filling the flask to
the mark with water.
Calculate the concentration in mol/L of the chemist's potassium iodide solution. Round your answer to 2 significant digits.
Please Help me!


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

You know the right answer?
A chemist prepares a solution of potassium iodide (KI) by measuring out 88. umol of potassium iodide...
Questions



Social Studies, 02.10.2020 09:01


Mathematics, 02.10.2020 09:01


Law, 02.10.2020 09:01

Mathematics, 02.10.2020 09:01



Mathematics, 02.10.2020 09:01

Mathematics, 02.10.2020 09:01



Spanish, 02.10.2020 09:01