
Chemistry, 24.01.2021 21:40 enthusiastfranklin
How many grams of hydrogen are produced from the reaction of 8.50 moles of zinc with an excess of hydrochloric acid? Zn+2HCl = ZnCl2+H2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
How many grams of hydrogen are produced from the reaction of 8.50 moles of zinc with an excess of hy...
Questions

Mathematics, 02.06.2021 21:30


Mathematics, 02.06.2021 21:30


English, 02.06.2021 21:30

Mathematics, 02.06.2021 21:30

Mathematics, 02.06.2021 21:30


Mathematics, 02.06.2021 21:30

Chemistry, 02.06.2021 21:30

Mathematics, 02.06.2021 21:30



Chemistry, 02.06.2021 21:30

English, 02.06.2021 21:30

Mathematics, 02.06.2021 21:30


English, 02.06.2021 21:30

English, 02.06.2021 21:30

Computers and Technology, 02.06.2021 21:30

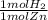 = 8.5 mol H2
= 8.5 mol H2 = 17.136 g H2
= 17.136 g H2

