
Chemistry, 25.01.2021 18:00 kromaug7986
2) If 51.3 grams of oxygen are used in this reaction, what mass of aluminum oxide could be produced?
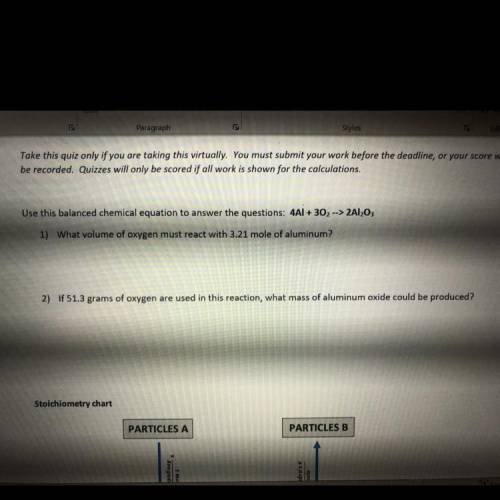

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
2) If 51.3 grams of oxygen are used in this reaction, what mass of aluminum oxide could be produced?...
Questions




Computers and Technology, 25.04.2020 01:01




Mathematics, 25.04.2020 01:01







Geography, 25.04.2020 01:01




Mathematics, 25.04.2020 01:01



