Chemistry, 14.10.2019 00:50 lnbrown5633
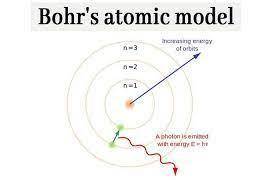
Scientists made the following two observations about emission spectra: each element has a unique emission spectrum. atoms emit energy only at specific wavelengths. describe how the bohr model explains both of these observations.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
The study of witch tree monkeys feed in is part of the science life
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1
You know the right answer?
Scientists made the following two observations about emission spectra: each element has a unique em...
Questions

Mathematics, 16.11.2020 19:20


Health, 16.11.2020 19:20

Mathematics, 16.11.2020 19:20

Social Studies, 16.11.2020 19:20

Mathematics, 16.11.2020 19:20


Mathematics, 16.11.2020 19:20

Mathematics, 16.11.2020 19:20

Mathematics, 16.11.2020 19:20

French, 16.11.2020 19:20

Mathematics, 16.11.2020 19:20

History, 16.11.2020 19:20


Physics, 16.11.2020 19:20

Biology, 16.11.2020 19:20

Mathematics, 16.11.2020 19:20


Mathematics, 16.11.2020 19:20