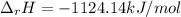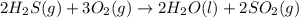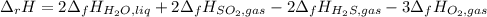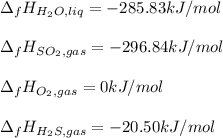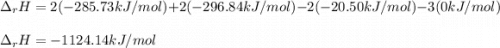Chemistry, 25.01.2021 20:20 coreyrineer2791
Using standard heats of formation, calculate the standard enthalpy change for the following reaction. 2H2S(g) 3O2(g)2H2O(l) 2SO2(g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1
You know the right answer?
Using standard heats of formation, calculate the standard enthalpy change for the following reaction...
Questions

Mathematics, 10.11.2020 18:20


Mathematics, 10.11.2020 18:20


Mathematics, 10.11.2020 18:20


Social Studies, 10.11.2020 18:20

Mathematics, 10.11.2020 18:20




Geography, 10.11.2020 18:20


Mathematics, 10.11.2020 18:20

Mathematics, 10.11.2020 18:20

English, 10.11.2020 18:20

History, 10.11.2020 18:20


Mathematics, 10.11.2020 18:20

Mathematics, 10.11.2020 18:20