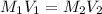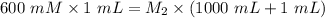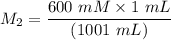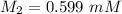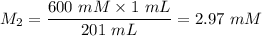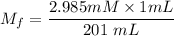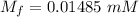Chemistry, 25.01.2021 20:50 kawaiiblurainbow
A purified protein is in a HEPES buffer at pH 7.0 with 600 mM NaCl. A 1 mL sample of the protein solution is dialyzed against 1.0 L of the same HEPES buffer with 0 mM NaCl. Once the dialysis has come to equilibrium, what is the concentration (in mM) of NaCl in the protein sample

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2


Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
A purified protein is in a HEPES buffer at pH 7.0 with 600 mM NaCl. A 1 mL sample of the protein sol...
Questions



Social Studies, 20.01.2021 07:50


Mathematics, 20.01.2021 07:50

Physics, 20.01.2021 07:50

Physics, 20.01.2021 07:50

Mathematics, 20.01.2021 07:50

Mathematics, 20.01.2021 07:50

English, 20.01.2021 07:50



History, 20.01.2021 07:50


Mathematics, 20.01.2021 07:50

Mathematics, 20.01.2021 07:50



Mathematics, 20.01.2021 07:50

English, 20.01.2021 07:50