L
10) A pure sample of an element was vaporized and injected into a mass spectrometer
and the...

Chemistry, 25.01.2021 21:40 stacywashburnstu
L
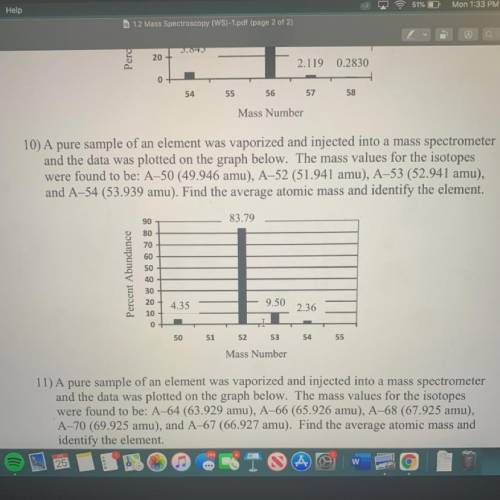
10) A pure sample of an element was vaporized and injected into a mass spectrometer
and the data was plotted on the graph below. The mass values for the isotopes
were found to be: A–50 (49.946 amu), A-52 (51.941 amu), A-53 (52.941 amu),
and A-54 (53.939 amu). Find the average atomic mass and identify the element.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1


Chemistry, 23.06.2019 04:31
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3
You know the right answer?
Questions

Computers and Technology, 18.01.2021 19:40

Mathematics, 18.01.2021 19:40



Mathematics, 18.01.2021 19:40

Physics, 18.01.2021 19:40




Mathematics, 18.01.2021 19:40



Medicine, 18.01.2021 19:40





Computers and Technology, 18.01.2021 19:40

Mathematics, 18.01.2021 19:40

English, 18.01.2021 19:40



