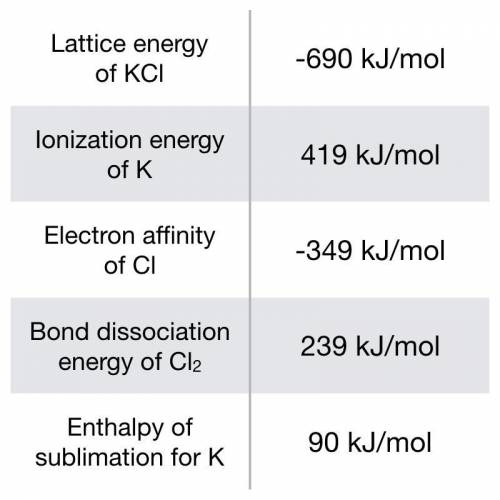
Using the following data, estimate ∆Hf° for potassium chloride: K(s) + ½ Cl₂(g) → KCl(s).
...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2
You know the right answer?
Questions

Mathematics, 10.10.2019 04:30




Mathematics, 10.10.2019 04:30


Mathematics, 10.10.2019 04:30


English, 10.10.2019 04:30


English, 10.10.2019 04:30

Mathematics, 10.10.2019 04:30

World Languages, 10.10.2019 04:30



Arts, 10.10.2019 04:30

Biology, 10.10.2019 04:30

Mathematics, 10.10.2019 04:30


Biology, 10.10.2019 04:30