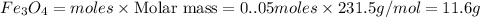Given the equation:
4Al2O3 + 9Fe --> 3Fe3O4 + 8Al
If 27.5 g of Al2O3 reacted with 8....

Chemistry, 26.01.2021 01:10 lazavionadams81
Given the equation:
4Al2O3 + 9Fe --> 3Fe3O4 + 8Al
If 27.5 g of Al2O3 reacted with 8.4 g of Fe, how many of Fe 3O4 are formed?
a) Calculate the limiting reactant
b) Calculate the number of grams of Al produced.
c) Calculate the number of grams of Fe3O4 produced.
d) Calculate the percent yield if 10g of Fe O4 were obtained?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
Questions



Mathematics, 12.07.2019 03:00

Mathematics, 12.07.2019 03:00

English, 12.07.2019 03:00

Mathematics, 12.07.2019 03:00

Mathematics, 12.07.2019 03:00

Mathematics, 12.07.2019 03:00

Biology, 12.07.2019 03:00

Advanced Placement (AP), 12.07.2019 03:00

Mathematics, 12.07.2019 03:00

Mathematics, 12.07.2019 03:00


Mathematics, 12.07.2019 03:00

English, 12.07.2019 03:00


Chemistry, 12.07.2019 03:00


History, 12.07.2019 03:00

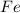 is the limiting reagent
is the limiting reagent 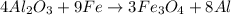
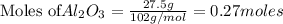
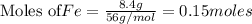
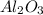
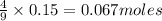 of
of 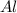
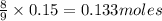 of
of 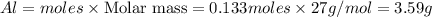
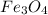
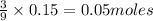 of
of