
Chemistry, 26.01.2021 02:00 tobyfoerst
A chemist mixed two substances together. Their repeating groups of atoms are shown below on the left. After they were mixed, the chemist analyzed the results and found two substances. Did a chemical reaction occur? Is the ending substance the same substance as the blue powder? What happened to the atoms of the starting substances when the ending substances formed? Be sure to explain your answers to both of these questions.
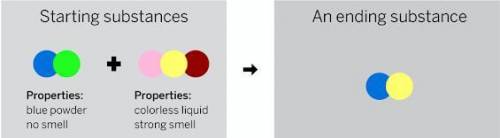

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2


You know the right answer?
A chemist mixed two substances together. Their repeating groups of atoms are shown below on the left...
Questions

Social Studies, 14.11.2019 06:31




Social Studies, 14.11.2019 06:31


Mathematics, 14.11.2019 06:31

Mathematics, 14.11.2019 06:31





Mathematics, 14.11.2019 06:31

Mathematics, 14.11.2019 06:31


Mathematics, 14.11.2019 06:31

Mathematics, 14.11.2019 06:31



Mathematics, 14.11.2019 06:31



