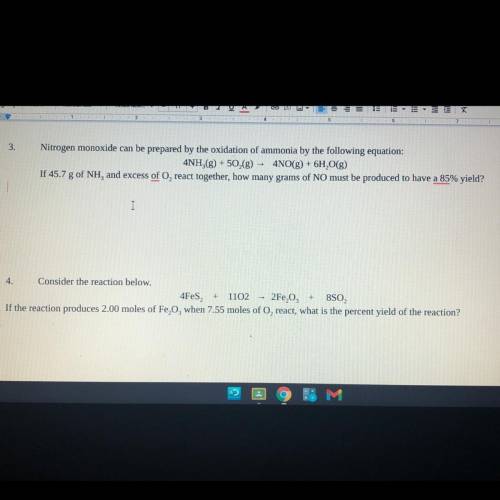
3. 68.55 g
4. 145.99%
Explanation:
3. Determination of the actual yield of NO.
4NH₃ + 5O₂ —> 4NO + 6H₂O
Next, we shall determine the mass of NH₃ that reacted and the NO that reacted from the balanced equation. This is illustrated below:
Molar mass of NH₃ = 14 + (3×1)
= 14 + 3
= 17 g/mol
Mass of NH₃ from the balanced equation = 4 × 17 = 68 g
Molar mass of NO = 14 + 16
= 30 g
Mass of NO from the balanced equation = 4 × 30 = 120 g
Summary:
From the balanced equation above,
68 g of NH₃ reacted to produce 120 g of NO.
Next, we shall determine the theoretical yield of NO. This can be obtained as follow:
From the balanced equation above,
68 g of NH₃ reacted to produce 120 g of NO.
Therefore, 45.7 g of NH₃ will react to produce = (45.7 × 120)/68 = 80.65 g of NO.
Thus, the theoretical yield of NO is 80.65 g
Finally, we shall determine the actual yield of NO. This can be obtained as follow:
Percentage yield of NO = 85%
Theoretical yield of NO = 80.65 g
Actual yield of NO =?
Percentage yield = Actual yield /Theoretical yield × 100
85% = Actual yield / 80.65
85/100 = Actual yield / 80.65
0.85 = Actual yield / 80.65
Cross multiply
Actual yield of NO = 0.85 × 80.65
Actual yield of NO = 68.55 g
4. Determination of the percentage yield.
4FeS₂ + 11O₂ —> 2Fe₂O₃ + 8SO₂
From the balanced equation above,
11 moles of O₂ reacted to produce 2 moles of Fe₂O₃.
Next, we shall determine the theoretical yield of Fe₂O₃. This can be obtained as follow:
From the balanced equation above,
11 moles of O₂ reacted to produce 2 moles of Fe₂O₃.
Therefore, 7.55 moles of O₂ will react to produce = (7.55 × 2)/11 = 1.37 moles of Fe₂O₃.
Thus, the theoretical yield of Fe₂O₃ is 1.37 moles.
Finally, we shall determine the percentage yield. This can be obtained as follow:
Actual yield of Fe₂O₃ = 2 moles
Theoretical yield of Fe₂O₃ = 1.37 moles
Percentage yield =?
Percentage yield = Actual yield /Theoretical yield × 100
Percentage yield = 2 / 1.37 ×100
Percentage yield = 200 / 1.37
Percentage yield = 145.99%