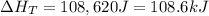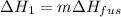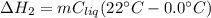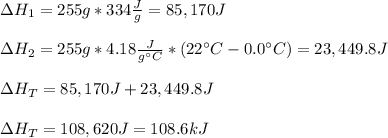Chemistry, 26.01.2021 06:20 pleasehelp5334me2
A 255 g sample of ice at 0.0 0C was melted and its temperature increased to 22 0C. What was the amount of heat (q) transferred?
Heat of fusion for water (ΔHfus) is 334 j/g
The specific heat of water is 4.18 J/g • 0C
This is a two step process. What steps are necessary to solve the problem?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3


Chemistry, 23.06.2019 06:30
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1

Chemistry, 23.06.2019 08:30
Sand is more likely than shale to preserve fossils. true false
Answers: 2
You know the right answer?
A 255 g sample of ice at 0.0 0C was melted and its temperature increased to 22 0C. What was the amou...
Questions

Geography, 25.03.2021 21:40

Mathematics, 25.03.2021 21:40




Mathematics, 25.03.2021 21:40













Geography, 25.03.2021 21:40