
Chemistry, 26.01.2021 08:10 scarlettlackey
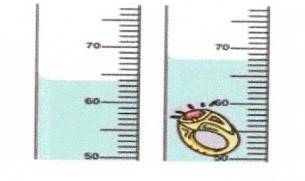
. Use Image B (picture above) and calculate the density of a ring that has a mass of 32 grams. Read Page 11 "Measure Volume Using Displacement" on your textbook before answering this question. Formula: D=m/V m=_ V= _ , then the density (D) is equal to D= _ This question requires three answers. Don't forget the units! *

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1


Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
. Use Image B (picture above) and calculate the density of a ring that has a mass of 32 grams. Read...
Questions





Mathematics, 22.01.2021 05:40

Mathematics, 22.01.2021 05:40

Mathematics, 22.01.2021 05:40

History, 22.01.2021 05:40

History, 22.01.2021 05:40

Mathematics, 22.01.2021 05:40

Mathematics, 22.01.2021 05:40





Mathematics, 22.01.2021 05:40


Mathematics, 22.01.2021 05:40

Mathematics, 22.01.2021 05:40

History, 22.01.2021 05:40



