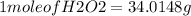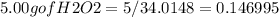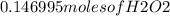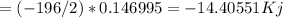Chemistry, 19.01.2020 09:31 calvinclifton
Hydrogen peroxide can decompose to water and oxygen by the following reaction
2h2o2 (l) 2h2o(l) + o2(g) enthalpy=-196kj
calculate the value of q when 5.00g of h20(l) decomposes at constant pressure.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2
You know the right answer?
Hydrogen peroxide can decompose to water and oxygen by the following reaction
2h2o2 (l)...
2h2o2 (l)...
Questions


Mathematics, 10.11.2019 21:31


Chemistry, 10.11.2019 21:31

Physics, 10.11.2019 21:31


History, 10.11.2019 21:31



Biology, 10.11.2019 21:31



History, 10.11.2019 21:31

Mathematics, 10.11.2019 21:31


Biology, 10.11.2019 21:31



Mathematics, 10.11.2019 21:31

Mathematics, 10.11.2019 21:31