The kp for the reaction below is 1.49 × 108 at 100.0°c:
co(g) + cl2(g) → cocl2(g)
...

The kp for the reaction below is 1.49 × 108 at 100.0°c:
co(g) + cl2(g) → cocl2(g)
in an equilibrium mixture of the three gases, pco = pcl2 = 2.22 × 10-4 atm. the partial pressure of the product, phosgene (cocl2), is atm.
a) 7.34
b) 3.02 × 10^15
c) 3.31 × 10^-16
d) 3.31 × 10^4
e) 6.67 × 10^11

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
Questions


English, 20.04.2021 22:10




History, 20.04.2021 22:10

History, 20.04.2021 22:10




Mathematics, 20.04.2021 22:10



History, 20.04.2021 22:10

English, 20.04.2021 22:10


Mathematics, 20.04.2021 22:10


Mathematics, 20.04.2021 22:10

English, 20.04.2021 22:10

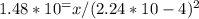 . So, the correct answer is A) 7.34.
. So, the correct answer is A) 7.34.

