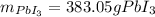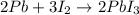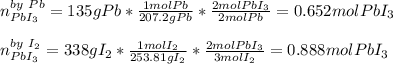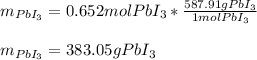Chemistry, 27.01.2021 06:50 Theresab2021
Calculate the mass of lead (III) iodide, Pb13, that can be produced by the reaction of 135 g of lead, Pb,
and 338 g of iodine.
1. What is the limiting reactant?
2. How much product is formed?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:10
Cobalt-60 is an artificial radioisotope that is produced in a nuclear reactor and is used as a gamma-ray source in the treatment of certain types of cancer. if the wavelength of the gamma radiation from a cobalt-60 source is 1.00 × 10-3 nm, calculate the energy of a photon of this radiation.
Answers: 2

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1
You know the right answer?
Calculate the mass of lead (III) iodide, Pb13, that can be produced by the reaction of 135 g of lead...
Questions




Mathematics, 03.04.2020 19:49




Mathematics, 03.04.2020 19:49

Mathematics, 03.04.2020 19:49

Mathematics, 03.04.2020 19:49



Mathematics, 03.04.2020 19:49



Biology, 03.04.2020 19:50

Mathematics, 03.04.2020 19:50

Arts, 03.04.2020 19:50

Mathematics, 03.04.2020 19:50