Using the model, write the chemical formula for an ionic compound that has Mg and Cl.
...

Chemistry, 27.01.2021 07:50 ChasityN8491
Using the model, write the chemical formula for an ionic compound that has Mg and Cl.
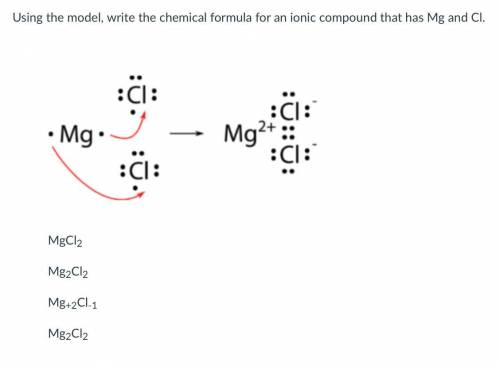

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
Questions

Mathematics, 24.05.2020 17:57

Mathematics, 24.05.2020 17:57


Mathematics, 24.05.2020 17:57


Mathematics, 24.05.2020 17:57

Mathematics, 24.05.2020 17:57



Business, 24.05.2020 17:57





English, 24.05.2020 17:57


Health, 24.05.2020 17:57


Mathematics, 24.05.2020 17:57



