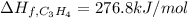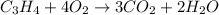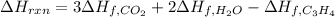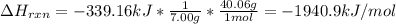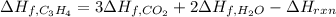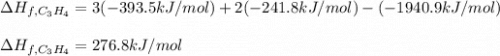Chemistry, 27.01.2021 09:40 Itsyaaboij4663
7.00 of Compound x with molecular formula C3H4 are burned in a constant-pressure calorimeter containing 35.00kg of water at 25c. The temperature of the water is observed to rise by 2.316c. (You may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) Calculate the standard heat of formation of Compound x at 25c. Be sure your answer has a unit symbol, if necessary, and round it to 3 significant digits.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
7.00 of Compound x with molecular formula C3H4 are burned in a constant-pressure calorimeter contain...
Questions


Advanced Placement (AP), 19.11.2019 04:31

Mathematics, 19.11.2019 04:31

Mathematics, 19.11.2019 04:31


Mathematics, 19.11.2019 04:31


Mathematics, 19.11.2019 04:31

English, 19.11.2019 04:31


Mathematics, 19.11.2019 04:31


Chemistry, 19.11.2019 04:31


Mathematics, 19.11.2019 04:31


Geography, 19.11.2019 04:31


Biology, 19.11.2019 04:31

History, 19.11.2019 04:31