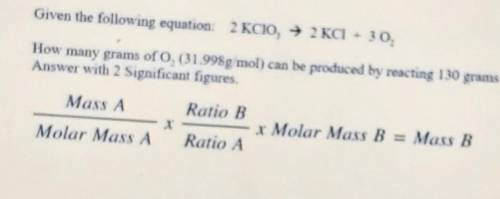Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
You know the right answer?
Given the following equation: 2KCIO, — 2 KCl + 30. How many grams of O, (ch31.998g mol) can be produ...
Questions

Mathematics, 02.11.2020 19:30



English, 02.11.2020 19:30

Mathematics, 02.11.2020 19:30


Mathematics, 02.11.2020 19:30

History, 02.11.2020 19:30




Mathematics, 02.11.2020 19:30



Mathematics, 02.11.2020 19:30