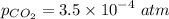Chemistry, 27.01.2021 19:50 exoticbunnylover123
The raw water supply for a community contains 18 mg/L total particulate matter. It is to be treated by addition of 60 mg alum (Al2(SO4)3 14H2O) per liter of water treated. Essentially, all the added alum precipitates represented by the following reaction:
Required:
a. For a total flow of 8000 m^3/d, compute the daily alum requirement and the concentration of solids in the water following alum addition, assuming the alum all precipitates as Al(OH)3(s).
b. The water is initially at pH 7.5 and has ALK=40 mg/L as CaCO3. It is desired to maintain solution pH at 6.5 or higher. Will the pH be in the acceptable range after the chemical addition and Al(OH)3(s) precipitation?
c. What will the pH be if the treated solution is bubbled with air, so that it reached equilibrium with atmospheric CO2?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

You know the right answer?
The raw water supply for a community contains 18 mg/L total particulate matter. It is to be treated...
Questions


Computers and Technology, 30.11.2021 03:00

SAT, 30.11.2021 03:00






History, 30.11.2021 03:00

History, 30.11.2021 03:00

English, 30.11.2021 03:00

Biology, 30.11.2021 03:00

SAT, 30.11.2021 03:00

Mathematics, 30.11.2021 03:00

Arts, 30.11.2021 03:00


Mathematics, 30.11.2021 03:00



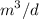

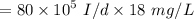
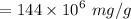

![$ [Al_2(SO_4)_3.14 H_2O] $](/tpl/images/1069/9830/978e1.png) required for one litre of the water treatment.
required for one litre of the water treatment.



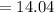 mg of alum ppt. per litre
mg of alum ppt. per litre


 ions.
ions. and water as follows :
and water as follows :