Consider the equations below.
CH4 (g)->C(s)+2H2(g) H1 = 74.6 kJ
C(s) + 2CI2(g)->CC...

Chemistry, 28.01.2021 06:40 zoelynn8386
Consider the equations below.
CH4 (g)->C(s)+2H2(g) H1 = 74.6 kJ
C(s) + 2CI2(g)->CCI4(g) H2 = -95.7 kJ
2H2(g) + 2CI2(g)-> 4HCI(g) H3 = -184.6 kJ
CH4(g) + 4CI2(g) -> CCI4(g) + 4HCI(g) H4 = -205.7 kJ
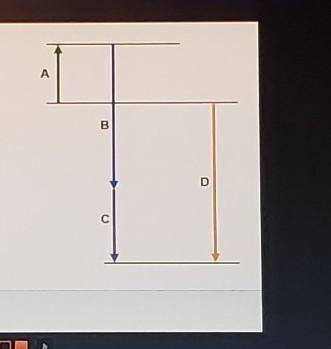
Complete the following based on the diagram.
Arrow A: 74.6 kJ
-95.7 kJ
-184.6 kJ
Arrow B: endothermic
exothermic
Arrow C: - bas a magnitude that is greater than that of B
- has a magnitude that is less than that of B
- has negative enthalpy
Arrow D: - represents an intermediate reaction
- has a magnitude that is always higher than any intermediate reaction
- represents the overall enthalpy of reaction

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
You know the right answer?
Questions



Physics, 28.02.2021 23:20

World Languages, 28.02.2021 23:20


Mathematics, 28.02.2021 23:20

Health, 28.02.2021 23:20

Mathematics, 28.02.2021 23:20

Mathematics, 28.02.2021 23:20

Mathematics, 28.02.2021 23:20

Mathematics, 28.02.2021 23:20

English, 28.02.2021 23:20






Mathematics, 28.02.2021 23:20


Mathematics, 28.02.2021 23:20



