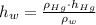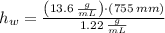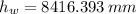Chemistry, 28.01.2021 22:20 Hazeleyes13
Suppose you were to construct a barometer using a fluid with a density of 1.22 g/mL. How high would the liquid level be in this barometer if the atmospheric pressure was 755 torr? (Mercury has a density of 13.6 g/mL.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2


Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

You know the right answer?
Suppose you were to construct a barometer using a fluid with a density of 1.22 g/mL. How high would...
Questions

Biology, 28.01.2020 15:49


Mathematics, 28.01.2020 15:49


Mathematics, 28.01.2020 15:49





English, 28.01.2020 15:49

Mathematics, 28.01.2020 15:49

Mathematics, 28.01.2020 15:49

Mathematics, 28.01.2020 15:49

Mathematics, 28.01.2020 15:49


Health, 28.01.2020 15:49



Social Studies, 28.01.2020 15:49

Geography, 28.01.2020 15:49

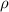 ), measured in grams per mililiter, and height of fluid (
), measured in grams per mililiter, and height of fluid (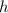 ), measured in milimeters. Two barometers with distinct fluids are equivalent when both have the same hydrostatic pressure. Then, we construct the following relationship:
), measured in milimeters. Two barometers with distinct fluids are equivalent when both have the same hydrostatic pressure. Then, we construct the following relationship: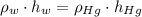 (1)
(1)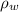 ,
, 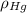 - Densities of fluid and mercury, measured in grams per mililiter.
- Densities of fluid and mercury, measured in grams per mililiter.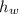 ,
, 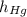 - Heights of fluid and mercury columns, measured in milimeters.
- Heights of fluid and mercury columns, measured in milimeters.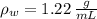 ,
, 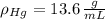 and
and 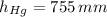 , then the liquid level of this barometer is:
, then the liquid level of this barometer is: