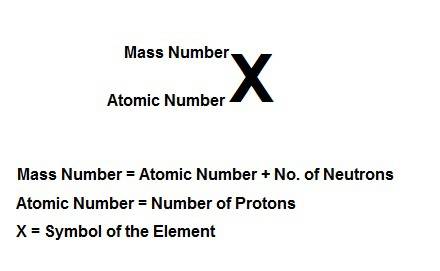
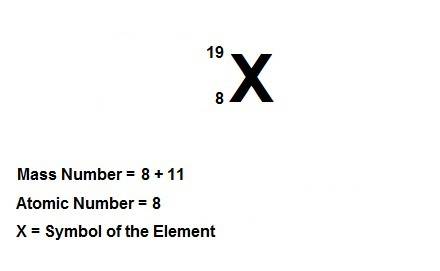Write the symbolic notation of an isotope of an element having 8 protons, 8 electrons, and 11 neutrons. click on the “templates” button template button and make use of the "stacked super/subscript" button button for entering stackes super/subscripts for entering the mass number and atomic number of the isotope. express your answer as an isotope.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1


Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1
You know the right answer?
Write the symbolic notation of an isotope of an element having 8 protons, 8 electrons, and 11 neutro...
Questions

Mathematics, 30.05.2020 10:57

World Languages, 30.05.2020 10:57

Mathematics, 30.05.2020 10:57

Mathematics, 30.05.2020 10:57


Mathematics, 30.05.2020 10:57





English, 30.05.2020 10:57

Social Studies, 30.05.2020 10:57

Mathematics, 30.05.2020 10:58

Biology, 30.05.2020 10:58


Mathematics, 30.05.2020 10:58

Medicine, 30.05.2020 10:58

Mathematics, 30.05.2020 10:58


Geography, 30.05.2020 10:58