2KClO3 2KCl + 3O2
2 - A. How many moles of oxygen gas would react with 250g of KCl?
2 -...

Chemistry, 29.01.2021 03:30 shortty1111
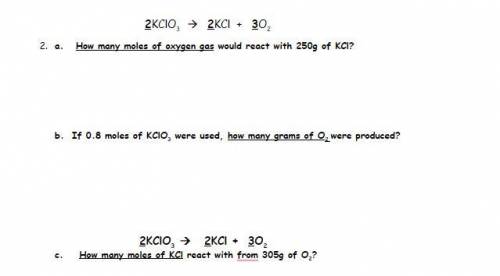
2KClO3 2KCl + 3O2
2 - A. How many moles of oxygen gas would react with 250g of KCl?
2 - B. If 0.8 moles of KClO3 were used, how many grams of O2 were produced?
2- C. How many moles of KCl react with 305g of O2?
There are multiple questions for the chemical equation, so I've just combined them all into one.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2


Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

You know the right answer?
Questions


English, 09.01.2021 14:00


Mathematics, 09.01.2021 14:00

Mathematics, 09.01.2021 14:00

Engineering, 09.01.2021 14:00


History, 09.01.2021 14:00

Spanish, 09.01.2021 14:00





Mathematics, 09.01.2021 14:00

English, 09.01.2021 14:00


Chemistry, 09.01.2021 14:00

History, 09.01.2021 14:00


Mathematics, 09.01.2021 14:00



