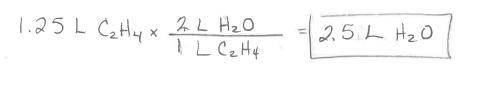Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 23.06.2019 02:00
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3

Chemistry, 23.06.2019 12:00
372 ml is the volume of aluminum, density is 2.70 g/ml what is the mass in grams
Answers: 1
You know the right answer?
Ethylene burns in oxygen to form carbon dioxide and water vapor:
C2H4(g) + 3 02(g) --> 2 CO2(g)...
Questions

Health, 10.12.2020 17:40


Biology, 10.12.2020 17:40

Social Studies, 10.12.2020 17:40



Mathematics, 10.12.2020 17:40



Physics, 10.12.2020 17:40


History, 10.12.2020 17:40


Mathematics, 10.12.2020 17:40

Chemistry, 10.12.2020 17:40

Business, 10.12.2020 17:40



English, 10.12.2020 17:40