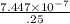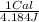Chemistry, 29.01.2021 05:30 elizabethann2
Assuming a 25% efficiency, how many Calories would a horse need to consume to work at 1.0 hp for 2.0 hh ?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
Assuming a 25% efficiency, how many Calories would a horse need to consume to work at 1.0 hp for 2.0...
Questions

Mathematics, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50



History, 12.12.2020 16:50


Computers and Technology, 12.12.2020 16:50



Mathematics, 12.12.2020 16:50


Biology, 12.12.2020 16:50






Computers and Technology, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50

Chemistry, 12.12.2020 16:50

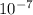 Cal
Cal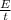 ...........1
...........1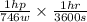
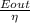 ..................2
..................2