
Chemistry, 29.01.2021 14:20 shaffergabe10
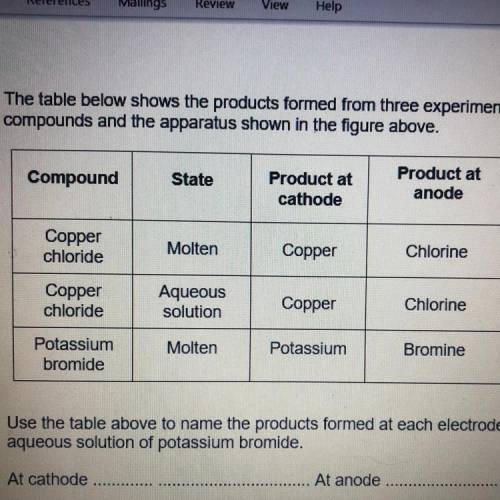
(b) The table below shows the products formed from three experiments using different
compounds and the apparatus shown in the figure above.
Compound
State
Product at
cathode
Product at
anode
Molten
Copper
Chlorine
Copper
chloride
Copper
chloride
Potassium
bromide
Aqueous
solution
Copper
Chlorine
Molten
Potassium
Bromine
Use the table above to name the products formed at each electrode if using an
aqueous solution of potassium bromide.
At cathode
At anode

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2

Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1
You know the right answer?
(b) The table below shows the products formed from three experiments using different
compounds and...
Questions

History, 04.02.2020 19:49


Mathematics, 04.02.2020 19:49







Geography, 04.02.2020 19:49

Geography, 04.02.2020 19:49

Mathematics, 04.02.2020 19:49

Social Studies, 04.02.2020 19:49

English, 04.02.2020 19:49

Chemistry, 04.02.2020 19:49


Social Studies, 04.02.2020 19:49

English, 04.02.2020 19:49

Business, 04.02.2020 19:49

Mathematics, 04.02.2020 19:49



