
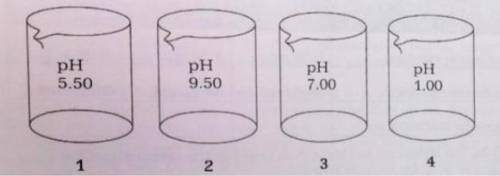
The four beakers above each contain 100.-mL of different solutions of similar concentrations.
(a) The Kb for ammonia is 1.8 x 10^-5
(i) Which beaker is most likely to contain NH3(aq)? Provide a chemical equation to explain your answer.
(ii) Calculate the molarity of the solution in the beaker that you chose for (i).
(b) If the contents of beakers 3 and 4 are poured together and mixed thoroughly, what will be the resulting pH?
(c) Explain how it is possible that beakers 1 and 4 are acids with equal molarities.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
You know the right answer?
The four beakers above each contain 100.-mL of different solutions of similar concentrations.
(a) T...
Questions

English, 09.04.2021 19:00


Social Studies, 09.04.2021 19:00


Mathematics, 09.04.2021 19:00

Mathematics, 09.04.2021 19:00

Health, 09.04.2021 19:00

Mathematics, 09.04.2021 19:00



Mathematics, 09.04.2021 19:00


History, 09.04.2021 19:00


History, 09.04.2021 19:00



Mathematics, 09.04.2021 19:00

Mathematics, 09.04.2021 19:00

Mathematics, 09.04.2021 19:00



