
Chemistry, 30.01.2021 05:40 hdjsjfjruejchhehd
An illustration of the Bohr model of the hydrogen atom is shown. The electron orbits are numbered 1-6 in orange and black. The black numbers are the wavelengths in nanometers of the associated electromagnetic radiation. Which wavelength is associated with a photon energy of 1.13 electron volts?
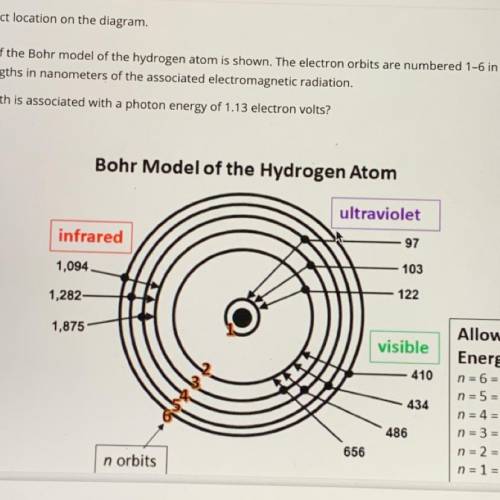

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
In the analysis of hair and fiber samples, which does a compound comparison microscope allow for that a conventional compound microscope does not? a. simultaneous observation b. polarization c. fluorescence d. higher magnification
Answers: 2

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

You know the right answer?
An illustration of the Bohr model of the hydrogen atom is shown. The electron orbits are numbered 1-...
Questions

Social Studies, 06.01.2020 18:31

Mathematics, 06.01.2020 18:31

Mathematics, 06.01.2020 18:31



SAT, 06.01.2020 18:31


English, 06.01.2020 18:31

Mathematics, 06.01.2020 18:31




Mathematics, 06.01.2020 18:31


Mathematics, 06.01.2020 18:31

English, 06.01.2020 18:31




Chemistry, 06.01.2020 18:31



